
DELIVERING HIGH QUALITY, ACCURATE
AND RAPID POINT OF CARE TESTING

Quantitative rapid tests
Our Products
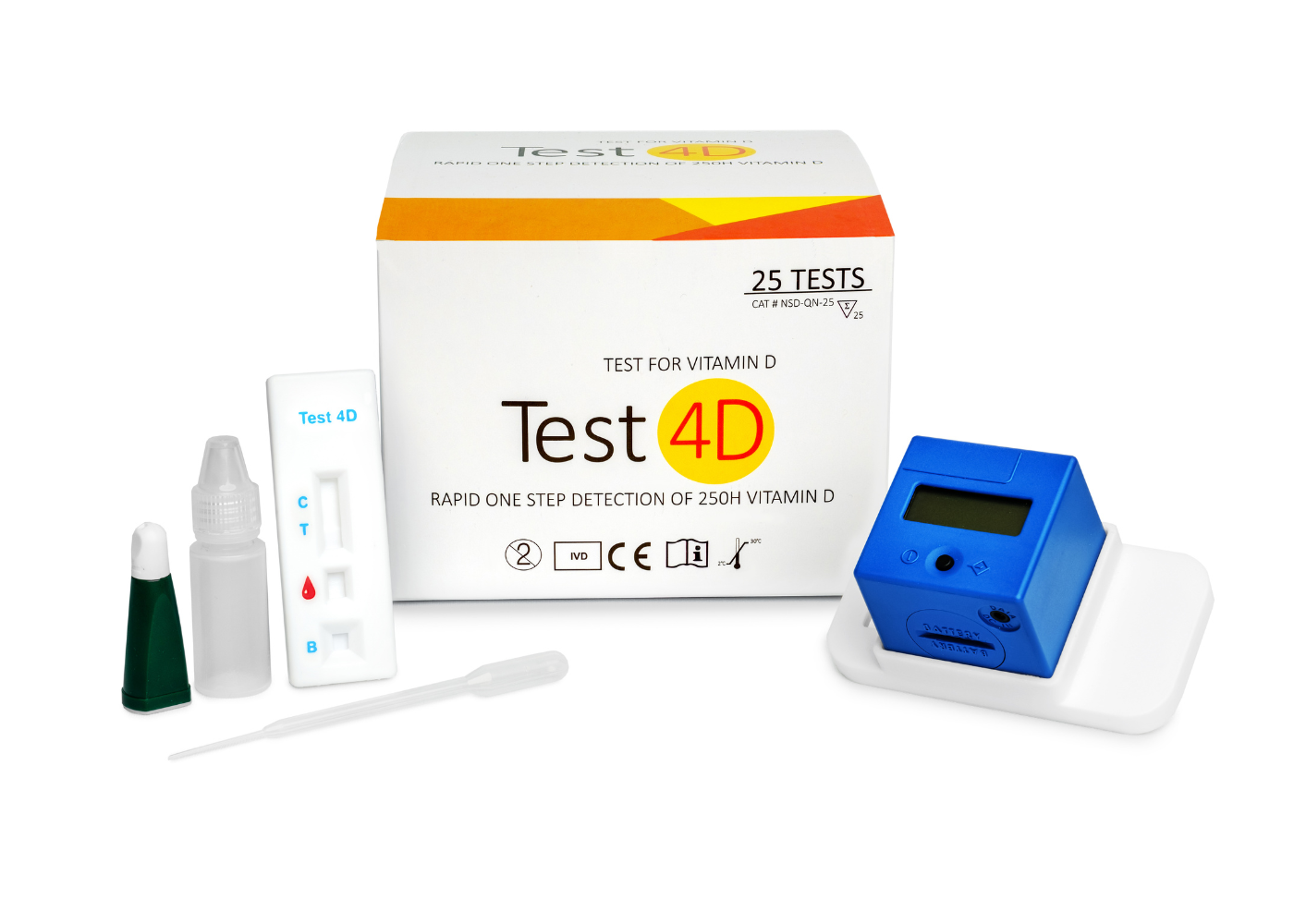
Test4D
Quantitative Vitamin D Test

Test4Fe
Quantitative Ferritin Test
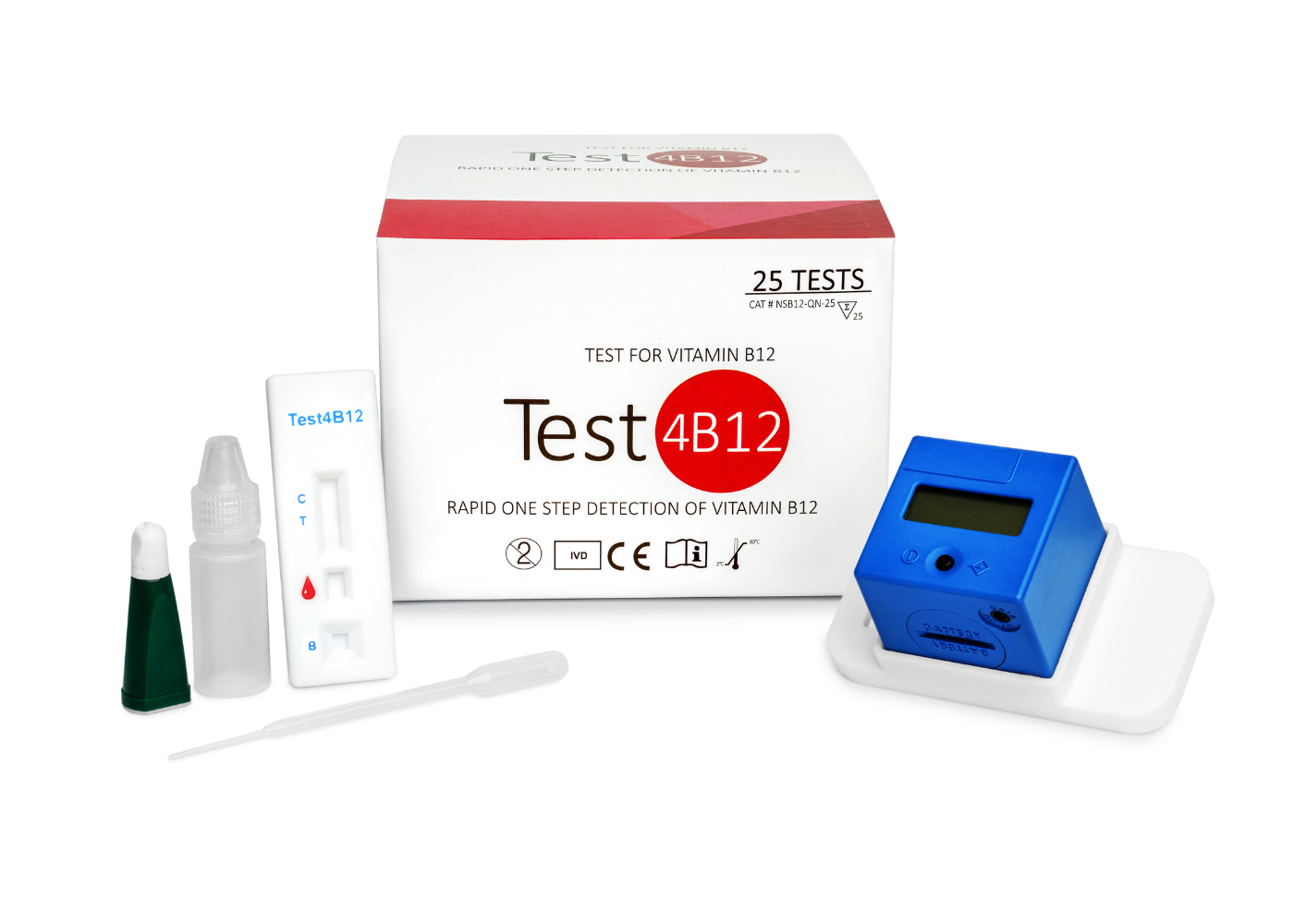
Test4B12
Quantitative Vitamin B12 Test
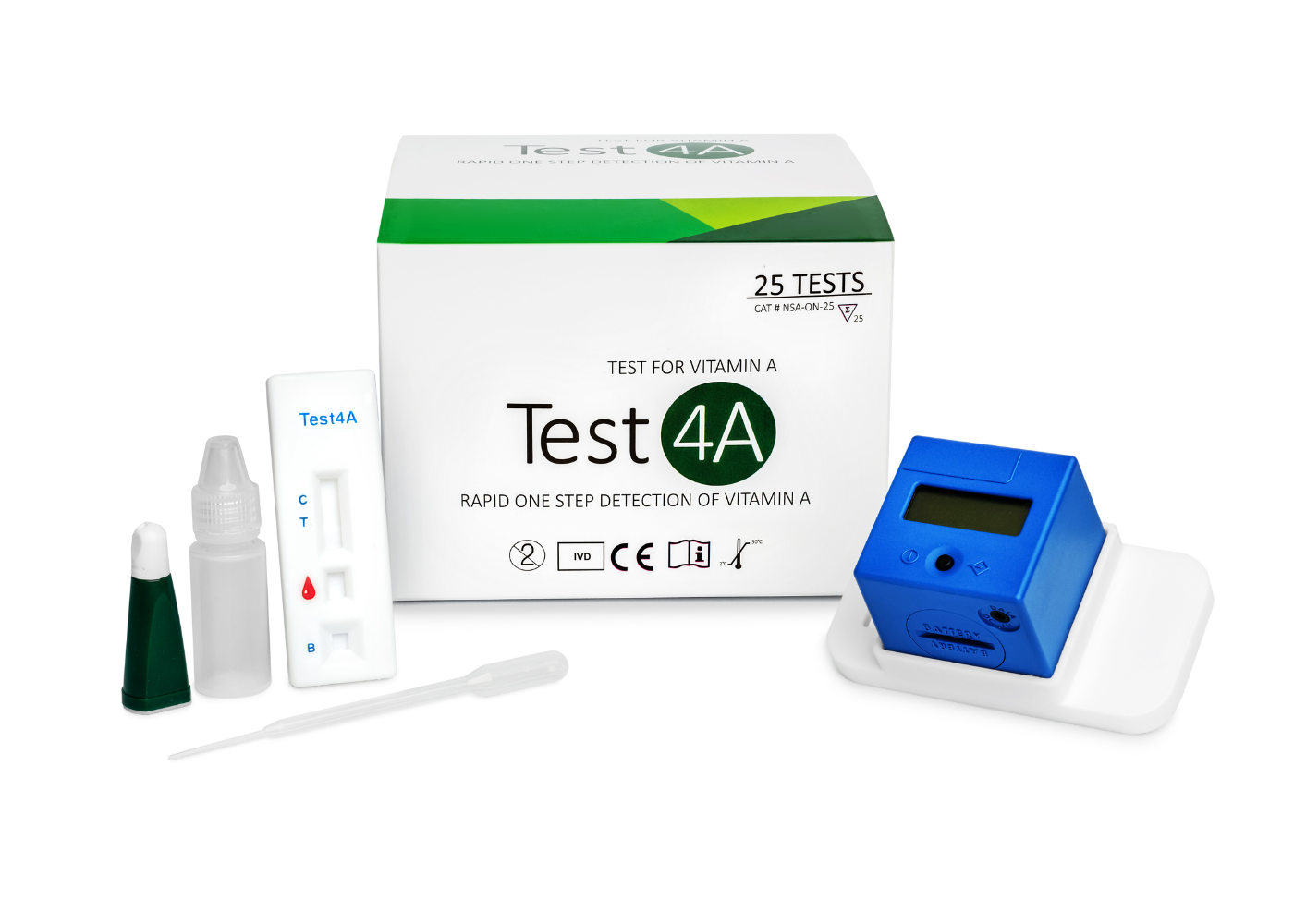
Test4A
Quantitative Vitamin A Test

Test4D
Quantitative Vitamin D Test

Test4Fe
Quantitative Ferritin Test

Test4B12
Quantitative Vitamin B12 Test

Test4A
Quantitative Vitamin A Test
Vitamin D test
Test4D
Quantitative Vitamin D test for fast and accurate results

Fast
Results in 10 minutes

Convenient
Easy to use

Reliable
Accurate and dependable results
Advanced Technology
Nanospeed features
Antibody
Proprietary patented monoclonal antibody is industry best practice

Cube Reader
Read values from sample in seconds
Sample
No sample prep

Precision
94% correlation with gold standard LC/MS

Importance of Vitamin D
1 Billion People are Vitamin D Deficient
Vitamin D is essential to your health.
Our testing kits can determine Vitamin D levels in as little as 10 minutes and are very simple to use
Our testing kits can determine Vitamin D levels in as little as 10 minutes and are very simple to use
Questions?
Contact us for any inquiries or support
